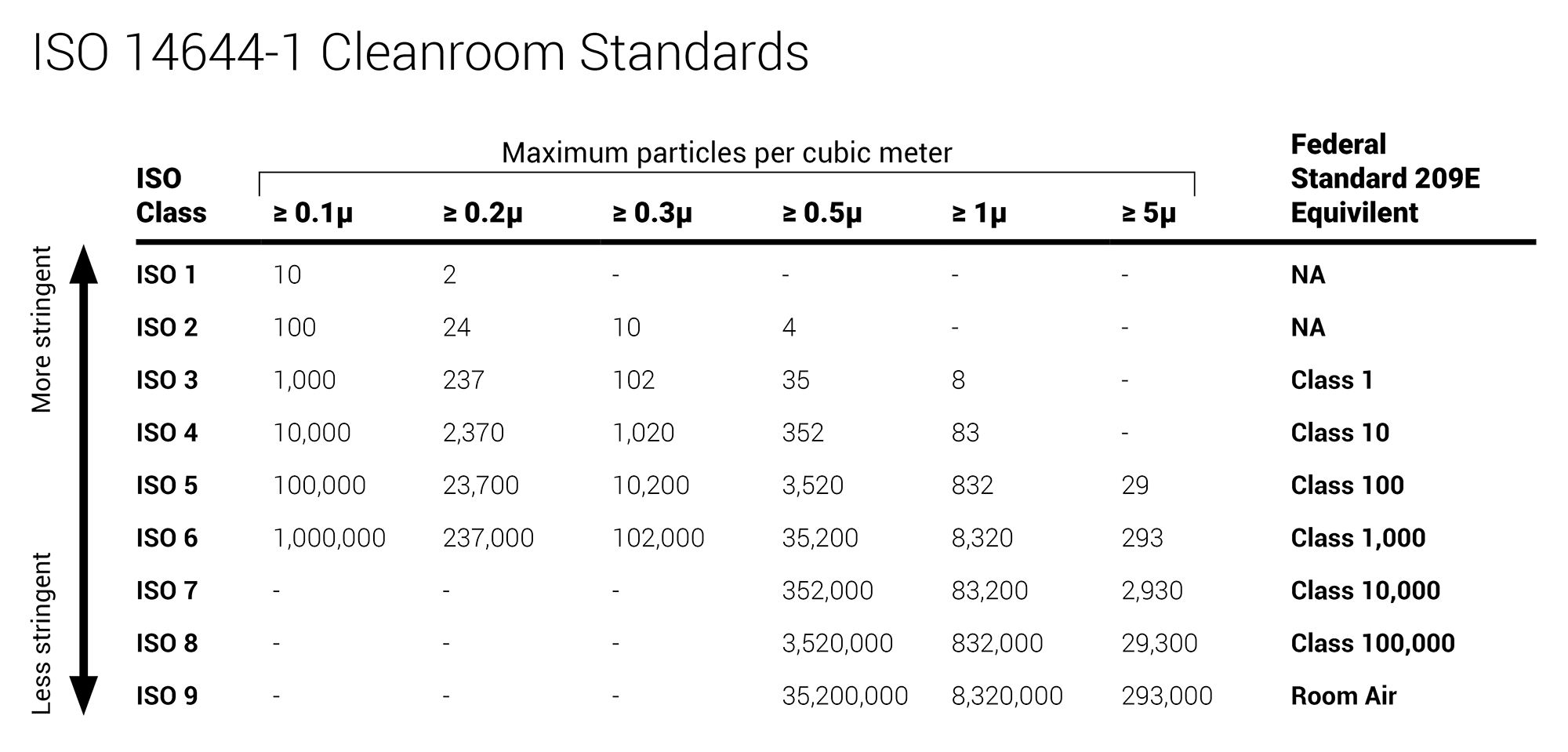
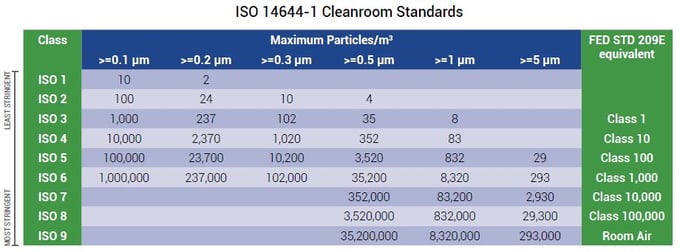
Iso classification of particulate matter in room air particles 0 5 µm and larger per cubic meter iso class particle count 3 35 2 4 352 5 3 520 6 35 200 7 352 000 8 3 520 000.
Usp 797 clean room humidity requirements.
United states pharmacopoeia usp 797 took effect on january 1st 2004 as a regulatory document which outlines procedures and environmental requirements for compounded sterile preparations csps.
Standards for compounding sterile preparations usp 797 helps to ensure patients receive quality preparations that are free from contaminants and are consistent in intended identity strength and potency.
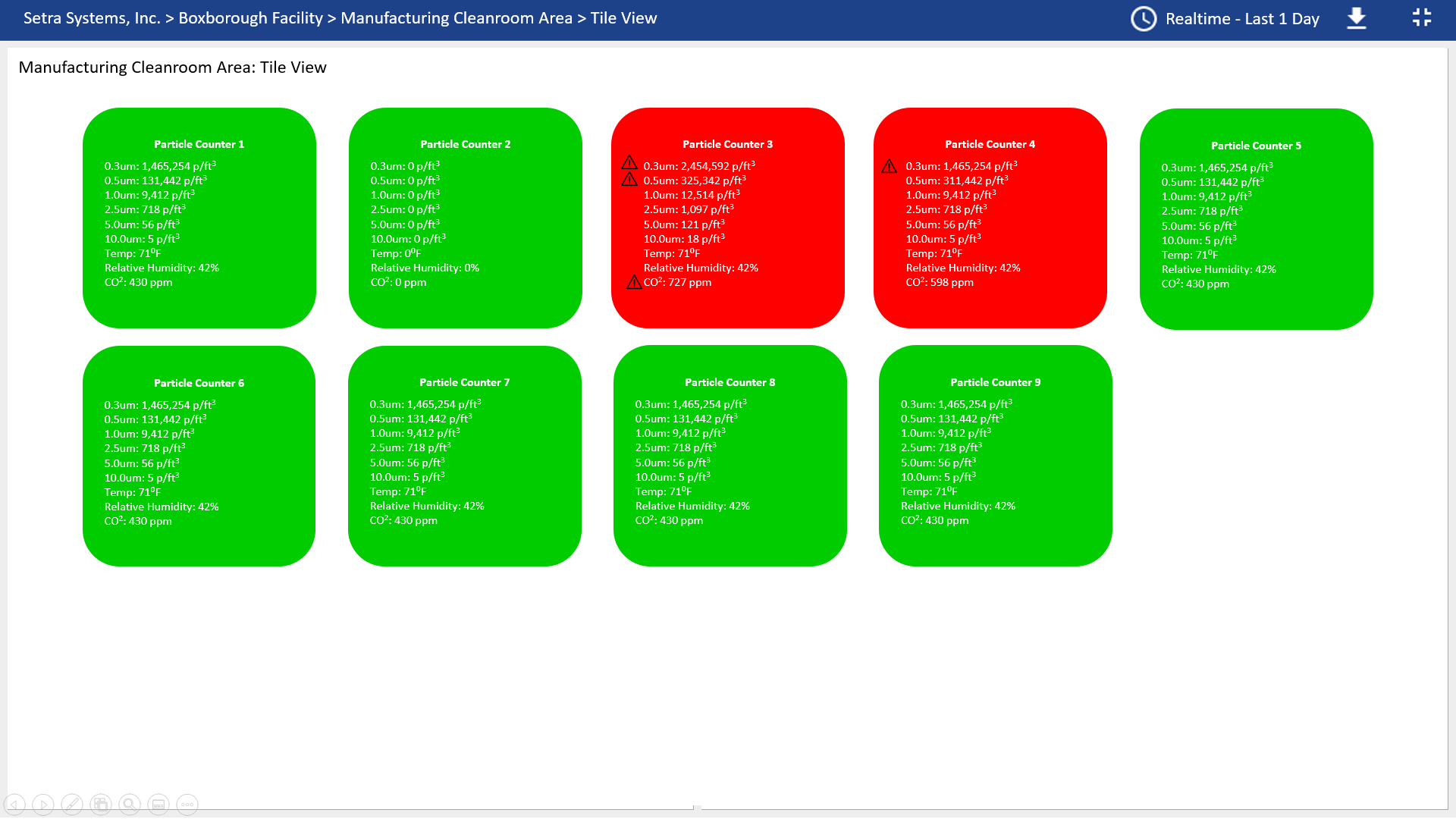
Implement a web based software system to ensure usp 797 compliance.
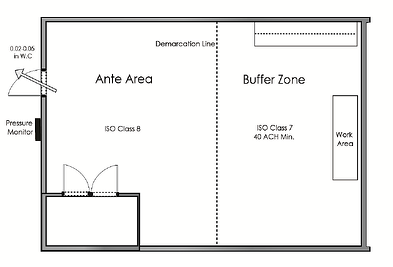
If the c sec for sterile compounding is a c sca the c sec should be externally vented.
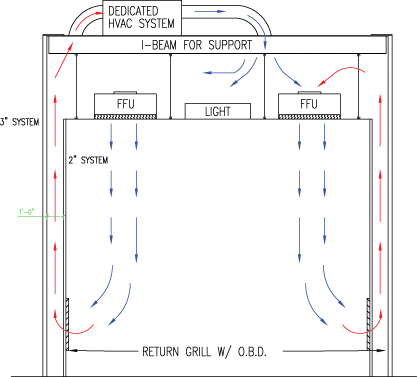
Favorable outcomes in usp 797 cleanrooms also require proper laminar flow workstation placement operator technique sanitation and room air cleanliness.
Ensure that your facility has a modern clean room environment.
Replace floors with seamless vinyl flooring.
The following discussion describes considerations for functional operation of the suite cleanroom design usp requirements and basic hvac principles that must be carefully melded together to design and build a usp 797 suite that can be certified as compliant.
It describes a number of requirements including responsibilities of compounding personnel training environmental monitoring.
Replace non compliant ceiling tiles with clean room grade tiles.
And be at negative pressure between 0 01 and 0 03 inches of water.
The iso class 7 ante room or non hd buffer room should maintain a positive pressure of at least 0 02 inches of water column to all adjacent unclassified areas usp 800 2016.
Usp 797 clean room guidelines standards portafab specializes in the turn key design and construction of modular clean rooms including applications specifically requiring usp 797 compliance.
Adopt usp 797 facility engineering clean room guidelines.